Generic Drugs: What They Are, Why They Work, and When to Watch Out
When you hear generic drugs, lower-cost versions of brand-name medications that contain the same active ingredients and meet the same FDA standards. Also known as generic medication, they make up over 90% of prescriptions in the U.S. because they work just as well—unless they don’t. That’s the catch. Most people assume switching to a generic is like swapping one brand of cereal for another. But for some drugs, especially those with a narrow therapeutic index, medications where even small changes in blood levels can cause serious side effects or treatment failure, the difference can be real. Drugs like warfarin, levothyroxine, and seizure meds fall into this category. A tiny shift in how your body absorbs the active ingredient can mean the difference between control and crisis.
Why does this happen? It’s not about quality—it’s about the invisible stuff. Generic drugs must match the brand in active ingredient, strength, and route of delivery. But they can use different fillers, dyes, or coatings. For most people, that’s fine. But if you’re sensitive to lactose, gluten, or certain dyes, those extras can trigger reactions. And if your body is finely tuned to a specific brand’s release pattern, switching to a generic with slightly different dissolution rates can throw off your balance. That’s why some patients report side effects after a switch: not because the generic is bad, but because their system is used to a particular version. Studies show that while most people see no change, a small but significant group do feel worse—especially with thyroid meds, epilepsy drugs, or blood thinners.
And it’s not just about biology. medication adherence, how consistently a patient takes their prescribed medicine drops when people don’t trust generics. Cultural beliefs, packaging changes, or even the color of a pill can make someone think they’re getting less. A patient who’s been on the same brand for years might see a new generic pill and assume it’s weaker—even if it’s identical. That’s why visual education tools like infographics help. Showing side-by-side comparisons, explaining FDA approval, and clarifying that generics must pass the same tests as brands builds trust. And trust keeps people on their meds.
So what should you do? If your doctor prescribes a generic, it’s usually safe. But if you’ve been stable on a brand-name drug and your pharmacy switches you without asking, speak up. Ask if your medication has a narrow therapeutic index. Check if your insurance forced the switch. Keep a log of how you feel after any change. And if you notice new side effects, worsening symptoms, or strange reactions—don’t ignore it. Talk to your pharmacist. They’re trained to spot these issues. You’re not being difficult. You’re being smart.
Below, you’ll find real stories and science-backed advice on what happens when people switch to generics, which drugs carry the highest risks, how culture shapes perception, and what to do if you feel off after a switch. This isn’t about fear. It’s about knowing when to question—and when to trust.
Learn how to ask your doctor about generic alternatives to save hundreds or even thousands on prescriptions without sacrificing effectiveness. Real savings, real science.
Feb, 9 2026
Generic drugs are safe for most people, but some patients report new side effects after switching. Is this due to real differences in formulation, psychological effects, or manufacturing quality? Here’s what the data says.
Feb, 1 2026
Bioavailability studies prove generic drugs work the same as brand-name versions by measuring how much and how fast the active ingredient enters the bloodstream. These tests are required for FDA approval and ensure safety, effectiveness, and cost savings.
Jan, 12 2026
Generic drugs are cost-effective and FDA-approved, but older adults face unique risks due to slower metabolism, polypharmacy, and increased sensitivity. Learn how to use them safely with the Beers Criteria and practical safety tips.
Jan, 11 2026
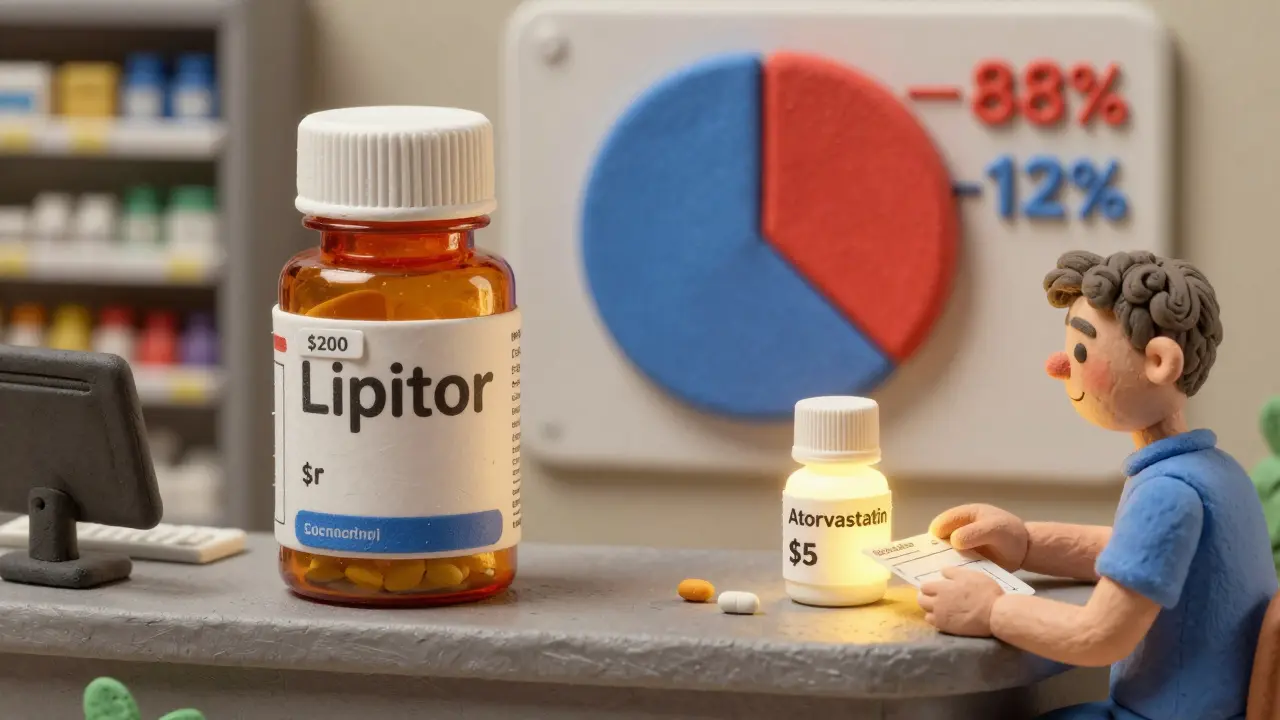
Generics make up 90% of U.S. prescriptions but only 12% of drug spending, saving billions annually. Learn how they work, why they’re cheaper, and why they’re the most effective tool for controlling healthcare costs.
Jan, 4 2026
Learn how narrow therapeutic index drugs work, why generic versions require extra caution, and what steps to take to stay safe when switching between brand and generic versions of critical medications.
Dec, 25 2025
Bioequivalence testing proves generic drugs work the same as brand-name versions by measuring how your body absorbs the active ingredient. It's the science behind 90% of U.S. prescriptions being generics.
Dec, 15 2025
Antitrust laws in the generic drug market prevent companies from blocking cheaper alternatives through pay-for-delay deals, patent manipulation, and product hopping. These practices cost patients billions and reduce access to essential medicines.
Nov, 28 2025