How Generics Control Healthcare Drug Spending in 2026
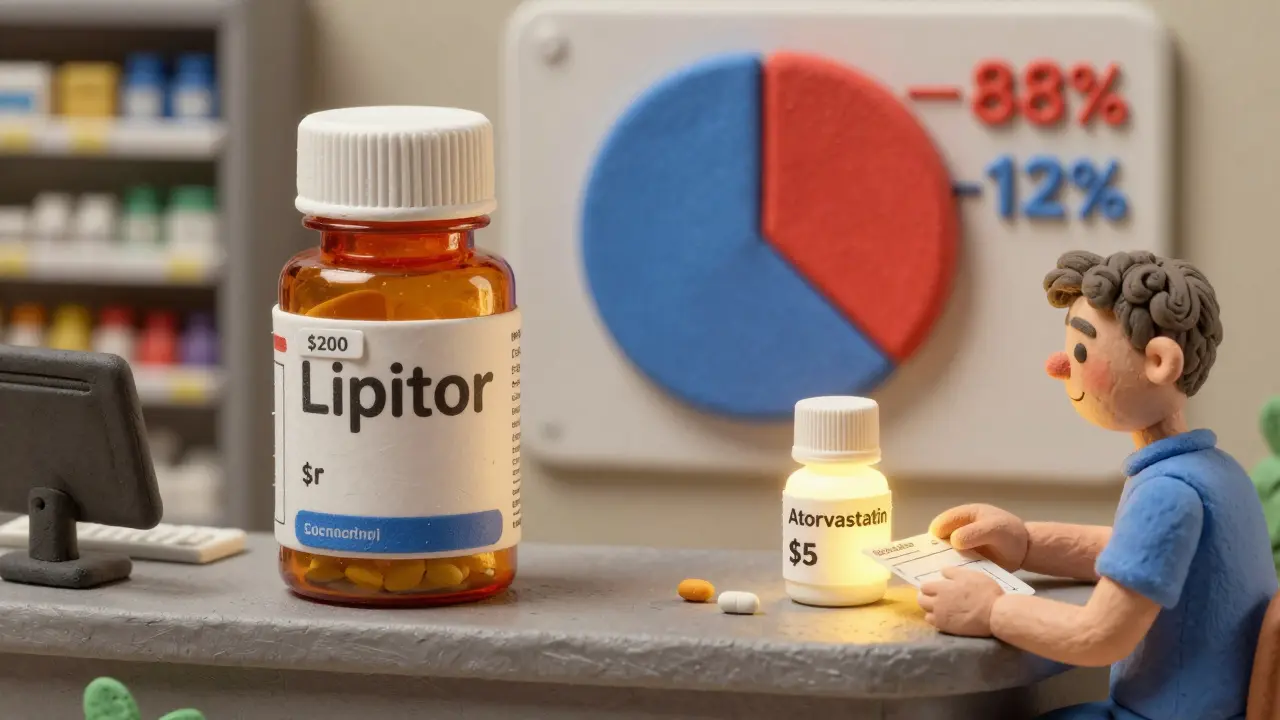
Every year, Americans spend over $650 billion on prescription drugs. That’s more than any other country in the world. But here’s the surprising part: 90% of all prescriptions filled are for generic drugs. And yet, those same generics make up only 12% of total drug spending. Meanwhile, brand-name drugs, which account for just 10% of prescriptions, soak up 88% of the money. This isn’t a glitch. It’s the system working as designed - and it’s all thanks to generic medications.
What exactly are generic drugs?
Generic drugs aren’t knockoffs. They’re exact copies of brand-name drugs, down to the active ingredient, strength, dosage form, and how they’re taken. The FDA requires them to be bioequivalent - meaning they work the same way in your body. That’s not theory. It’s science. Manufacturers must prove their version delivers the same amount of medicine into your bloodstream at the same rate as the original. They test this in 24 to 36 healthy volunteers, taking blood samples over 72 hours, using strict statistical models. If the results fall within 80-125% of the brand’s numbers, the FDA approves it.That’s why you can switch from brand-name Lipitor to atorvastatin and get the same cholesterol-lowering effect. Or swap out brand-name Metformin for the generic version and still control your blood sugar. The FDA’s Orange Book lists over 14,000 approved generic products, each with a therapeutic equivalence code. An ‘A’ rating means you can substitute it without worry.
Why do generics cost so much less?
Brand-name drugs aren’t just expensive because they’re fancy. They’re expensive because developing them costs an average of $2.6 billion and takes 10 to 15 years. That includes clinical trials, regulatory hurdles, and failed candidates. Generics skip most of that. Once a patent expires, a company can file an Abbreviated New Drug Application (ANDA) - a much simpler, faster process. The FDA doesn’t require new safety or efficacy trials. They just need proof that the generic matches the original.The result? Generics launch at 80-85% lower prices. In 2024, generics saved the U.S. healthcare system $98 billion in direct spending. That’s not a guess. It’s from the Association for Accessible Medicines’ official report. Cumulative savings since 2000 now exceed $445 billion. That’s money that went back into patients’ pockets, insurance premiums, or hospital budgets.
The rise of biosimilars - the next wave
Not all drugs are easy to copy. Biologics - drugs made from living cells, like Humira, Enbrel, or insulin - are complex. You can’t just mix chemicals in a lab. That’s where biosimilars come in. They’re not exact copies, but they’re highly similar, with no clinically meaningful differences in safety or effectiveness. They cost 15-35% less than the original biologic.But here’s the problem: adoption is slow. In Europe, 70-85% of biologic prescriptions are filled with biosimilars. In the U.S., it’s only 25-30%. Why? Pharmacy benefit managers (PBMs) often push brand-name biologics because they get bigger rebates. Even when a cheaper biosimilar exists, the formulary might not list it first. Patients don’t always know they have a cheaper option - and sometimes their doctor doesn’t either.
By 2025, the Congressional Budget Office estimates biosimilars could save the system $133 billion. But right now, 90% of biologics set to lose patent protection in the next decade have no biosimilar in development. That’s a massive missed opportunity.
Who’s making these drugs - and where?
The U.S. doesn’t make most of its generic drugs. About 80% of the active ingredients come from India and China. That’s not inherently bad - those countries produce high-quality, low-cost APIs. But it’s risky. During the pandemic, supply chain disruptions caused over 300 drug shortages, mostly affecting generics. When a single factory in India shuts down for quality issues, thousands of Americans can’t get their blood pressure or diabetes meds.The biggest generic manufacturers are Teva, Viatris, and Sandoz. But Chinese and Indian firms like Sun Pharma and Dr. Reddy’s control nearly half the global API market. The FDA inspects these facilities, but with over 400 foreign plants supplying U.S. generics, oversight is stretched thin. In 2023, the FDA’s Generic Drug Shortage Task Force flagged 127 drugs at risk of running out.
Why aren’t generics always used?
You’d think with such clear savings, everyone would use generics. But barriers exist.Patent thickets: Brand companies file dozens - sometimes over 140 - patents on one drug. Not all are about the medicine itself. Some cover packaging, dosing schedules, or delivery devices. These patents delay generics for years. The FTC found these tactics add an average of 17 months to generic entry.
Pay-for-delay deals: Sometimes, brand companies pay generic makers to stay out of the market. These illegal agreements cost consumers $3.5 billion a year, according to the FTC. Courts have cracked down, but they still happen.
Product hopping: A brand company makes a tiny change - say, switching from a pill to a tablet - and gets a new patent. Suddenly, the old version is off-patent, but the new one isn’t. Generics can’t enter until the new patent expires. That delays competition by 6-12 months.
Authorized generics: Sometimes, the brand company itself launches a generic version. It’s cheaper than the brand, but not as cheap as a true third-party generic. That keeps prices higher than they’d be with real competition.
Real people, real savings
Numbers matter, but so do stories. One Reddit user shared how switching their mother from brand-name Humalog insulin ($350/month) to generic insulin lispro ($25/month) kept her from rationing doses. That’s not hypothetical. GoodRx’s 2024 report found 68% of patients skip or split pills when generics aren’t available. Among Medicare Part D users, 42% skip doses for brand-name drugs - only 12% do so for generics.Patients aren’t just saving money. They’re staying alive. A Drugs.com analysis of 1.2 million reviews showed generics scored 4.1 out of 5 for overall satisfaction - nearly identical to brand-name drugs. Efficacy ratings were the same. But affordability? Generics scored 4.5 out of 5. Brands? 2.3.
Where generics fall short
Generics aren’t perfect. Some drugs are hard to copy.Narrow therapeutic index drugs like warfarin or levothyroxine require extremely precise dosing. Even tiny variations in absorption can cause problems. Some patients report side effects after switching - especially with levothyroxine. The FDA says generics are equivalent. But some doctors still prefer to stick with one brand, and patients sometimes do too.
Complex delivery systems - inhalers, injectables, topical creams - are harder to replicate. Bioequivalence testing gets messy. A generic inhaler might deliver the same drug, but if the particle size or spray pattern is off, the lung absorption changes. The FDA is still developing standards for these.
Orphan drugs - for rare diseases - rarely get generics. There’s just not enough market to justify the cost of development. That leaves patients with no affordable options.

What’s changing in 2026?
The Inflation Reduction Act capped insulin at $35/month for Medicare patients in 2023. That forced companies like Eli Lilly to drop their list prices from $275 to $25 - and that price drop spread to commercial markets too. It’s not a law forcing generics in - it’s a price shock that changed the whole market.The FDA’s 2024 Biosimilars Action Plan aims to cut approval times by 50%. That’s critical. Right now, it takes 10-12 months to approve a generic. For a biosimilar? Often over two years.
Medicare’s new drug price negotiation program will cut costs on 10 drugs in 2026 - but only those 10. The Congressional Budget Office says even if they expand to 30 drugs a year, the savings won’t match what generics already deliver. Why? Because negotiation only affects Medicare’s share. Generics affect everyone - Medicare, Medicaid, private insurers, cash payers.
What patients and doctors can do
You don’t need a policy change to save money. Start here:- Ask your doctor: “Is there a generic version?” Even if you’re on a brand, it might be off-patent.
- Check the FDA Orange Book for therapeutic equivalence codes. If it’s an ‘A’ code, substitution is safe.
- Use GoodRx or SingleCare to compare prices. Sometimes the cash price for a generic is cheaper than your copay.
- If you switch and feel different - report it. The FDA’s MedWatch system tracks adverse events. In 2023, over 1,200 reports came in from patients reacting to inactive ingredients in generics.
- Ask your pharmacist: “Can you substitute?” In 48 states, they can - unless your doctor says no.
Most insurance plans automatically switch to generics. But not all. Some commercial plans charge higher copays for generics than brand-name drugs - because the rebate structure favors the brand. That’s backward. Push back. Ask your employer or insurer why.
The bottom line
Generics aren’t a band-aid. They’re the backbone of affordable healthcare. They’re not perfect, but they’re the most powerful tool we have to control drug spending. Every time a generic enters the market, prices drop 90% within a year. Medicare negotiation? It drops prices by 42%. Generics win by a landslide.Biologics are the next frontier. But without faster approvals, stronger incentives for manufacturers, and an end to rebate distortions, we’ll keep paying too much for life-saving drugs.
Until then, the simplest, most effective thing you can do? Ask for the generic. It’s not just cheaper. It’s just as good.
Are generic drugs as effective as brand-name drugs?
Yes. The FDA requires generics to have the same active ingredient, strength, dosage form, and route of administration as the brand-name drug. They must also prove bioequivalence - meaning they deliver the same amount of medicine into your bloodstream at the same rate. Over 14,000 generic products are approved and monitored. Studies show identical effectiveness in real-world use, with patient satisfaction ratings nearly matching brand-name drugs.
Why are generic drugs so much cheaper?
Brand-name drugs cost billions to develop and take over a decade to get approved. Generics skip expensive clinical trials because they’re copying an already-approved drug. They only need to prove they’re bioequivalent, which costs about $1 million and takes 10-12 months. That’s why they launch at 80-85% lower prices. The savings come from cutting development costs, not cutting quality.
Can I trust generics made in India or China?
Yes. The FDA inspects all manufacturing facilities - foreign and domestic - that supply drugs to the U.S. About 80% of active ingredients come from India and China, and many of these factories meet the same standards as U.S. plants. The FDA has inspection teams in both countries and can refuse imports if standards aren’t met. While supply chain issues have caused shortages, quality control is rigorously enforced.
Why do some people say generics don’t work for them?
Most of the time, it’s not the active ingredient. It’s the inactive ingredients - fillers, dyes, or binders - which can differ between brands and generics. Some people have sensitivities to these. For example, lactose or gluten in a pill might cause stomach issues. This is rare, but real. The FDA received over 1,200 adverse event reports in 2023 related to these differences. If you feel different after switching, talk to your doctor. You might need to switch back or find a different generic.
Why aren’t more biosimilars available?
Biosimilars are harder and more expensive to develop than small-molecule generics. They require complex testing and can take years to get approved. Plus, pharmacy benefit managers often favor brand-name biologics because they get bigger rebates, even when a cheaper biosimilar exists. That means doctors and patients don’t always see the cheaper option. Only 25-30% of biologic prescriptions in the U.S. are filled with biosimilars - compared to 70-85% in Europe.
Does Medicare automatically use generics?
Yes. Over 98% of Medicare Part D plans automatically substitute generics when available. They’re built into the formulary. But commercial insurance plans are less consistent. Only 58% of private plans automatically substitute without extra cost to the patient. Some even charge higher copays for generics than brand-name drugs - a practice called "generic differentials." Always check your plan’s formulary.
What’s the biggest barrier to more generic use?
The biggest barrier isn’t science - it’s money. Brand companies use legal tactics like "pay-for-delay" deals and patent thickets to delay generic entry. Pharmacy benefit managers prioritize rebates over savings. And some doctors aren’t trained to recognize when substitution is safe. These aren’t medical issues - they’re economic and systemic ones. Fixing them requires policy changes, not just patient education.

Terri Gladden
January 5, 2026 AT 09:04Jennifer Glass
January 5, 2026 AT 12:13en Max
January 7, 2026 AT 05:30Angie Rehe
January 8, 2026 AT 13:06josh plum
January 9, 2026 AT 10:30John Ross
January 11, 2026 AT 08:18Ashley Viñas
January 12, 2026 AT 01:57Brendan F. Cochran
January 12, 2026 AT 19:29Mandy Kowitz
January 13, 2026 AT 00:44